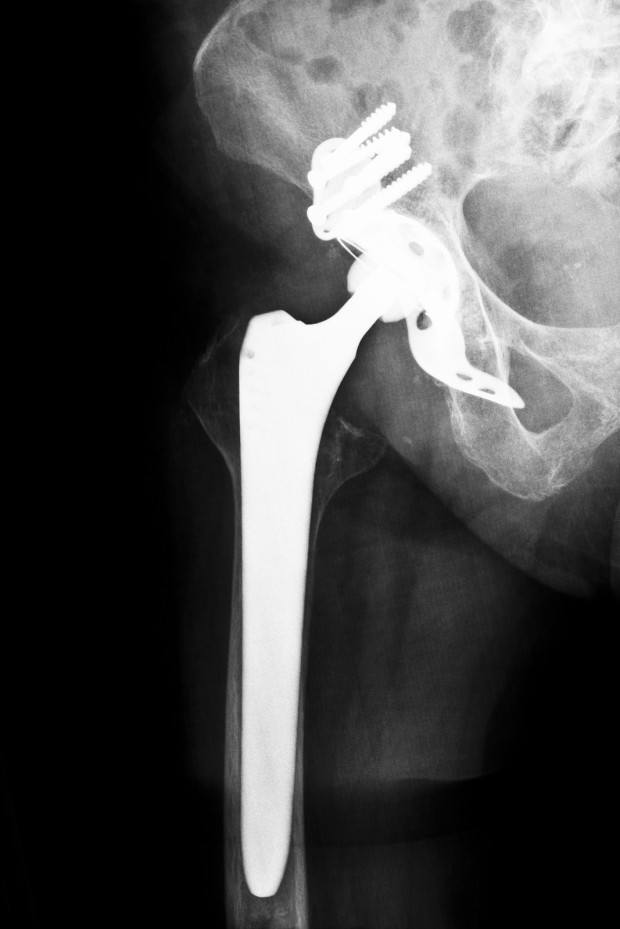
Last Tuesday, an Illinois jury found in favor of Johnson & Johnson’s DePuy orthopedics unit in a second product liability trial of nearly 11,000 lawsuits over the company’s recalled ASR metal hip implant.
The outcome contrasts with an earlier separate trial in which a Los Angeles jury ordered the J&J unit to pay more than $8 million in damages to plaintiff Loren Kransky after finding the company’s ASR hip was defectively designed. DePuy is appealing that verdict.
While litigation relating to the metal hips is expected to be expensive, some analysts have said that J&J, one of the world’s largest healthcare companies with annual revenue of about $70 billion, should be able to weather the costs.
The Illinois jury awarded no damages to plaintiff Carol Strum, J&J said in a statement. Strum, a nurse who claimed her ASR hip was defective, received the implant in 2008 and had it removed in 2011.
J&J voluntarily recalled the product from the market in August 2010, in response to data that showed some ASR patients were undergoing a second hip replacement surgery sooner than expected. The company said about 10,750 plaintiffs have direct claims in pending lawsuits over the product. About 93,000 ASR hips were sold worldwide prior to the recall.
“DePuy’s actions concerning the product were appropriate and responsible, including the program to address patients’ medical costs related to the recall,” said Lorie Gawreluk, a spokeswoman for DePuy.
Calls to attorneys for the plaintiff were not immediately returned.
All-metal implants were developed to be more durable than traditional hip implants, which combine a ceramic or metal ball with a plastic socket, but doctors became concerned about the devices after they were shown to fail more often. The all-metal hips can shed metal debris where two components connect, potentially damaging surrounding soft tissue.
The U.S. Food and Drug Administration earlier this year issued a proposal calling on companies that make all-metal hip replacements to provide additional information proving they are safe and effective before being allowed to continue selling them.
Cases filed in U.S. federal courts have been consolidated in the U.S. District Court for the Northern District of Ohio. That trial is set to begin in June.




















 Focus on Ski Guides After Deadly California Avalanche Could Lead to Criminal Charges, Civil Suits
Focus on Ski Guides After Deadly California Avalanche Could Lead to Criminal Charges, Civil Suits  Is Risk the Main Ingredient in Ultra-Processed Food?
Is Risk the Main Ingredient in Ultra-Processed Food?  Reinsurance Program Could Wipe Out Need for Calif. FAIR Plan: Legal Exec
Reinsurance Program Could Wipe Out Need for Calif. FAIR Plan: Legal Exec  AI Claim Assistant Now Taking Auto Damage Claims Calls at Travelers
AI Claim Assistant Now Taking Auto Damage Claims Calls at Travelers 



